

The thermochemical hydrogen cycle has been successfully tested in Japan, however only in glassware and not at the high temperatures expected from the GT-MHR reactor. Even so, the results have been very positive, according to Schultz, with Japanese researchers running two loops -- one at about 10 liters of hydrogen per hour, and a second loop at 30 liters per hour. Both loops ran for approximately one week.
“The Japan Atomic Energy Research Institute at Oarai has demonstrated the whole process, says Schultz. “All done at one atmosphere and thus at temperatures that are not prototypical of what you’d do in a real production plant, so the efficiency doesn’t count. This is a proof of principle, not necessarily a proof of practicality,” he adds.
Schultz explains that the Japanese are also planning a pilot-scale experiment to be constructed in the next year or two, which is expected to produce 30 cubic meters of hydrogen per hour. “Here in the U.S., we are proceeding along the same lines as the Japanese, except we’re doing it at full operating conditions -- full pressure and full temperature conditions. The folks at Sandia National Laboratory have demonstrated the sulfuric acid decomposition,” Schultz explains, while General Atomics has been working on the hydrogen iodide decomposition.
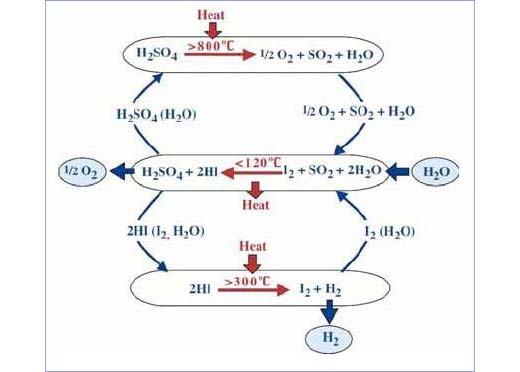
A special still vaporizes and then decomposes the hydrogen iodide, condensing out the iodine and separating the hydrogen for collection. “This uses pretty exotic materials, says Schultz. “Tantalum and tungsten are necessary for use for the hydrogen iodide; we use phosphoric acid to separate the hydrogen iodide from the water and we decompose the pure hydrogen iodide in a still.”
Taking the individual demonstration projects that Sandia, General Atomics and the CEA lab in Saclay, France, have been developing, the pieces will be brought together at General Atomics to create a single unit to demonstrate the complete process at full temperature and pressure.
“It would produce something in the range of 200 to 1,000 liters of hydrogen per hour, says Schultz, “which is relatively modest by vehicle standards -- a tenth of a kilogram per hour. That’s our laboratory-scale demonstration,” he continues.
“We hope to finish the construction of this by the end of this year, and then do experiments with it, testing particularly the control of these three reactions in two loops that have to be able to work together in synchrony. We’ve got to figure out how to be able to control the three such that they do work in synchrony.”
Whereas the thermochemical method of hydrogen production scales directly with volume, electrolysis does not. Because of this, Schultz maintains that the thermochemical process is intrinsically more efficient than either low- or high-temperature electrolysis.
Entire contents © 2006 Corland Publishing. Use of editorial content without permission is strictly prohibited.
All Rights Reserved. Privacy Policy Legal Contact Us. Site developed by ICON.




