

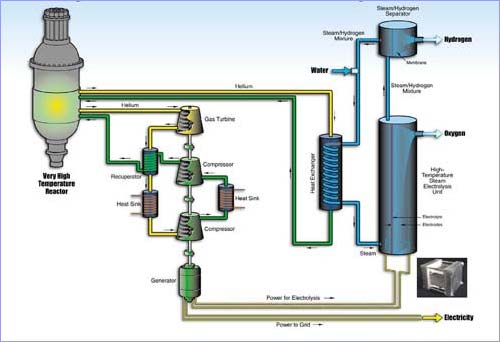
High-temperature Electrolysis
From the standpoint of hydrogen production, the GT-MHR’s electrical generating capability combined with its 950-degree Celsius operating temperature is well suited to high-temperature electrolysis. This process is much more efficient than low-temperature electrolysis due several factors, including lower capital costs from reduced precious metal content in the catalyst.
“One thing that we are avoiding is the use of inherently expensive materials,” Herring reminds. “We don’t want to use any platinum and we want to use nickel as little as possible. We’re using stainless rather than using platinum or nickel.”


Dr. S. Elangovan of Ceramatec, and Dr. Steve Herring, INL, hold one of Ceramatec’s high-temperature electrolyzer stacks. High-temperature electrolysis is 50-percent more efficient than low-temperature processes.
Especially interesting to researchers is the greater efficiency of high-temperature electrolysis. “As you go to higher temperature, you need less electricity and you’re replacing that energy with heat,” Herring explains. “So as you go from 100 degrees to about 900 degrees Celsius, the amount of electricity goes down by about 20 percent or so. This allows us to have much better efficiency than we would have in a conventional electrolyzer.”
High-temperature electrolysis employs both electricity and heat to separate the hydrogen and oxygen atoms out of water. “We use some of the heat for producing high-temperature steam in a heat exchanger, and most of the heat for producing electricity via a breaking cycle,” explains Herring.
Within the electrolyzer, steam at 800-degrees Celsius loses the oxygen atoms, as they pass though the 0.15mm-thick membrane. The steam/hydrogen mixture then travels through a porous membrane, allowing relatively pure hydrogen to come out. “This whole loop operates then at about 800 degrees, with the exception of the liquid water that’s coming in,” says Herring.

Ceramatec’s electrolyzer stacks use a high-temperature ceramic membrane just 150 microns thick and made from yttrium, or scandia-stabilized zirconia.
Herring explains that a 25-cell stack has been tested for 1,000 hours, producing on average about 177 normal liters of hydrogen per hour. “We have a test that is running right now that has gone for almost 700 hours,” he adds. “It’s a dual-stack and we’re still operating with the 10-centimeter square cells. Sometime in the future we’re going to go to a larger size,” Herring adds.
Currently, Ceramatec has recorded 800 normal liters of hydrogen per hour from the 120-cell parallel stack, according to Herring. Ceramatec will subsequently move to a quad arrangement using four 60-cell modules. “Our next step will be an integrated lab cell experiment, which is quad modules,” he states.
Ultimately, three of these quad units will be assembled, together with the other necessary components to create a scaled-down test unit able to fit on a 6-by-8-foot skid -- the integrated laboratory-scale unit will be built at the Idaho National Engineering Laboratory and is expected to produce up to 5,000 normal liters per hour.
“We would incorporate all of the equipment that would go with a reactor plant,” explains Herring. “All of the heat exchangers, oxygen and hydrogen separation equipment, steam generators and water purification equipment.”

Using a parallel arrangement, this 120-cell Ceramatec test stack has operated successfully for 690 hours. A laboratory-scale three-gang, quad arrangement is expected to produce 8000 normal liters of hydrogen per hour.
At the current stage of research, the cost per kilowatt is about $10,000, according to Ceramatec officials. But this cost will drop considerably in actual use. “We have seen studies for volume production of $400 or $500 per kilowatt,” says Herring. Of course, the overall cost will be affected by the operating life, which Ceramatec is predicting (based on its experience with solid oxide fuel cells) at approximately 40,000 hours -- five years of continuous operation.
Entire contents © 2006 Corland Publishing. Use of editorial content without permission is strictly prohibited.
All Rights Reserved. Privacy Policy Legal Contact Us. Site developed by ICON.




